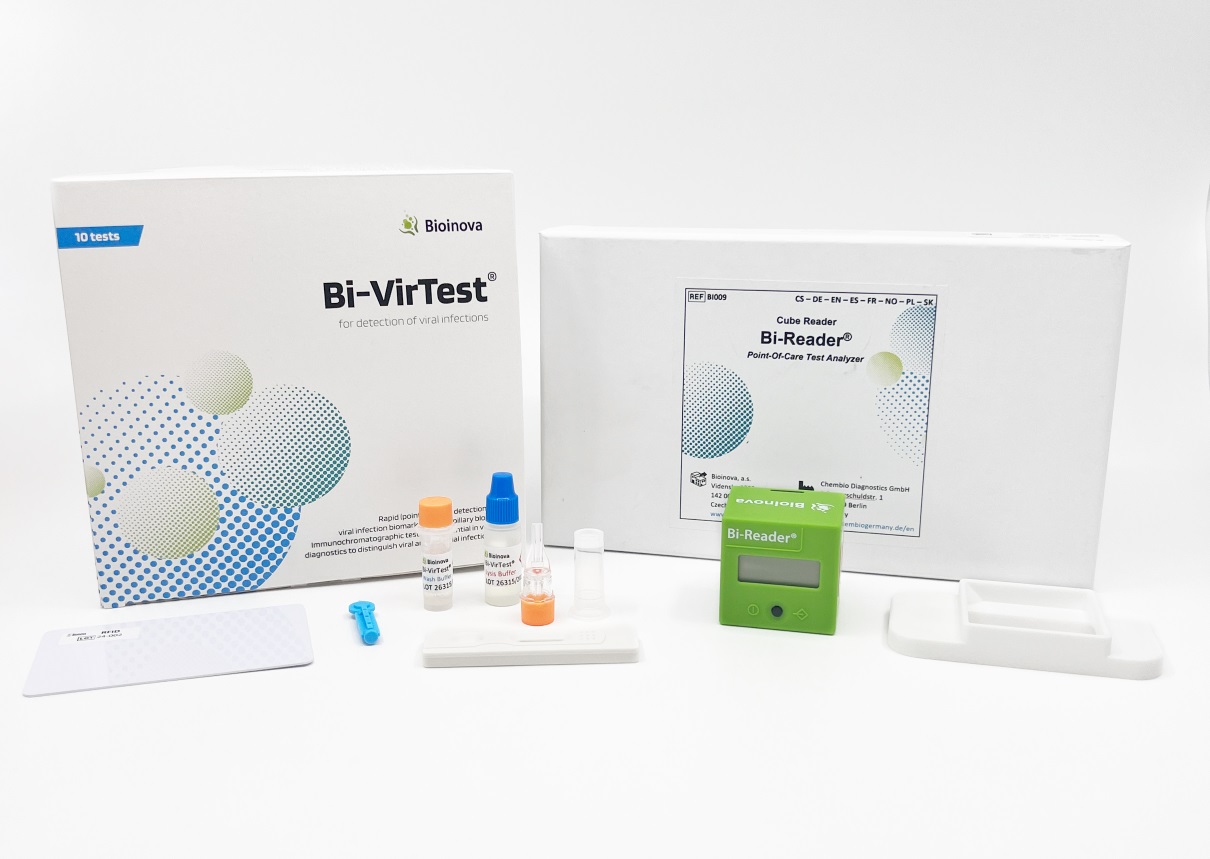
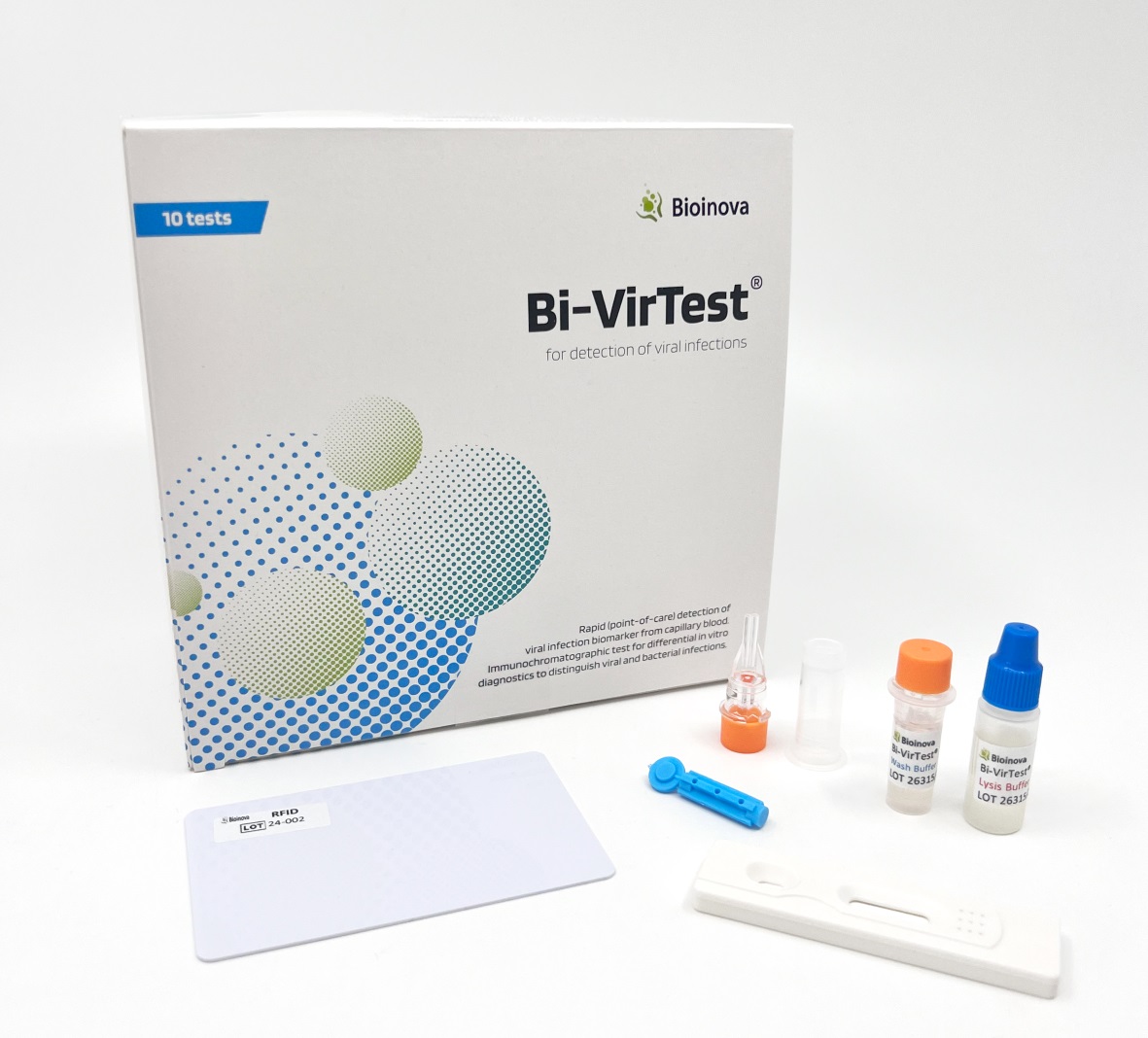
Bi-VirTest for Human MxA POCT
- Bilder (3)
| Artikelname: | Bi-VirTest for Human MxA POCT |
| Artikelnummer: | BVD-BI005-10 |
| Hersteller Artikelnummer: | BI005-10 |
| Alternativnummer: | BVD-BI005-10 |
| Hersteller: | BioVendor |
| Kategorie: | Kits/Assays |
| The CE IVD-certified Bi-VirTest is a sensitive and specific rapid test for the early detection of viral infections. |
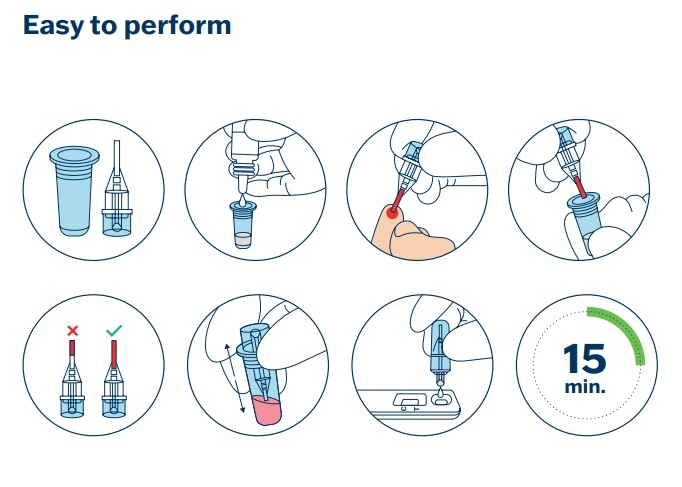
BioVendor Group presents MxA protein, a unique biomarker of viral activity, which allows the differentiation between viral and bacterial infections for effective antibiotic treatment. The CE IVD-certified Bi-VirTest is a sensitive and specific rapid test for the early detection of viral infections. Together with the LFT-Reader (Art. No. BVD-BI-Reader), the Bi-VirTest enables precise quantitative measurement of the MxA level in blood samples (capillary blood). The MxA level allows reliable conclusions to be drawn about the presence of a viral infection.
Regulatory Status of the Bi-VirTest for Human MxA (POCT)
Research topicImmune Response, Infection and Inflammation, Sepsis, COVID-19
BackgroundHuman MxA protein (Myxovirus resistance protein 1), encoded by the MX1 gene, is a 76-kDa protein composed of 662 amino acid residues and is a member of the dynamin superfamily of large GTPases. The MxA protein plays a crucial role in antiviral defense within cells, providing protection against a broad range of viruses, including influenza, parainfluenza, measles, coxsackie, hepatitis B, and Thogoto viruses. The viruses are inhibited by MxA protein at an early stage in their life cycle, soon after host cell entry and before genome amplification. The human MxA protein is accumulated in the cytoplasm and endoplasmic reticulum. The membrane compartment of endoplasmatic reticulum seems to provide an interaction platform that facilitates viral target recognition. MxA appears to detect viral infection by sensing and trapping nucleocapsid structures, and becoming the viral components unavailable for the generation of new virus particles.The expression of viral MxA protein is induced exclusively and, in a dose-dependent manner, by IFN-alpha and IFN-beta, but not by IFN-gamma, IL-1, TNF-alpha or other cytokines. MxA protein may offer advantages as a laboratory marker because of its very low basal concentration, increasing within 1-2 hours of infection and long half-life being approximately 2-3 days. In mononuclear cells stimulated with high doses of leukocyte IFN-alpha, MxA mRNA levels increased tenfold within 4 hours, and elevated MxA protein levels persists over the next 48 hours. MxA protein with its low basal concentration and long half-life, offers advantages as a marker for viral infection. Clinical studies have reported on MxA protein in peripheral blood mononuclear cells as a marker distinguishing viral from bacterial disease, and as a reliable marker for type I IFN bioavailability during IFN treatment in patients with multiple sclerosis (MS) according to the recommendation from EMA (European Medicine Agency).
Main Clinical Use of MxA
Technical DataType: Lateral Flow Test Description: Differentiation between viral and bacterial infections; Viral infections: acute phase disease monitoring Quantitative Measuring Range: 5-200 ng/ml Cut-off: 21 ng/ml Clinical Sensitivity and Specificity: ≥ 93% and 95% Assay Time: 15 minutes Applications: Capillary Blood |