![]()
|
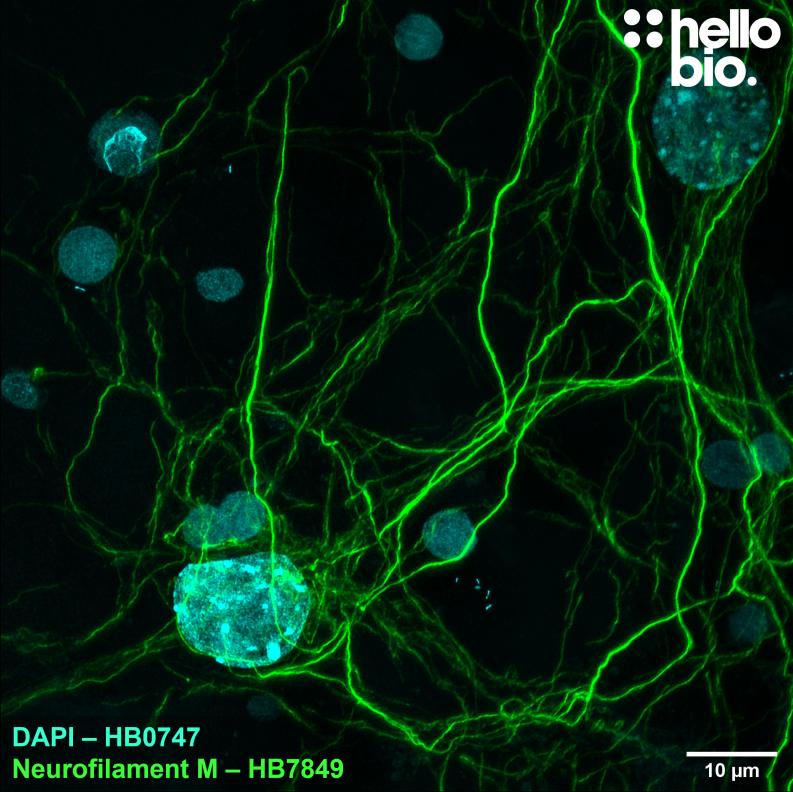
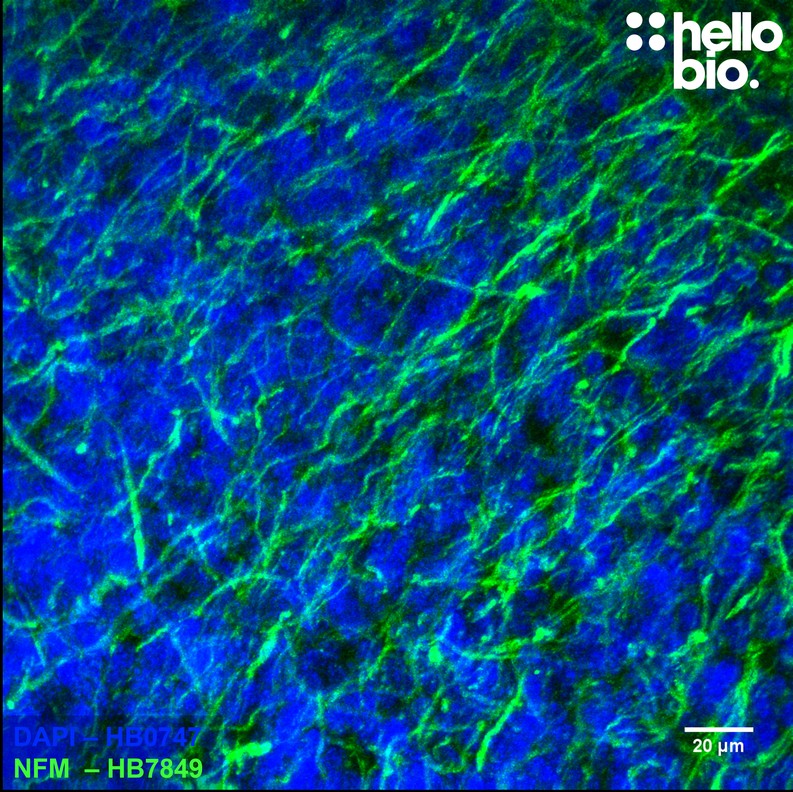
Figure 1. Neurofilament M expression in cultured rat neurones visualised using HB7849. HB7849 visualised the dense neurofilament network of cultured rat neurones. Method: neurones were cultured from PND2 rats following established protocols (Brewer and Torricelli, 2007. Nat Protoc 2, 1490–1498) and fixed with 4% PFA on DIV21. Cells were permeabilised with 0.1% Triton X-100 followed by blocking in 1% BSA, 300mM glycine. HB7849 was incubated overnight (4°C) at a 1:1000 dilution (1µg/ml) followed by a one hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our ICC protocol. Images were captured using a Leica SPE confocal laser scanning microscope coupled to a Leica DMi8 inverted epifluorescence microscope. The image was captured using a 63x objective, 405nm (15.0% power, gain: 618) and 488nm (35.1% power, gain: 684) laser lines in a z-stack (0.35 µm spacing). Deconvolution was carried out using Huygens Essential (Scientific Volume Imagine) followed by the stack being flattened using a maximum Z projection in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682). |
![]()
|
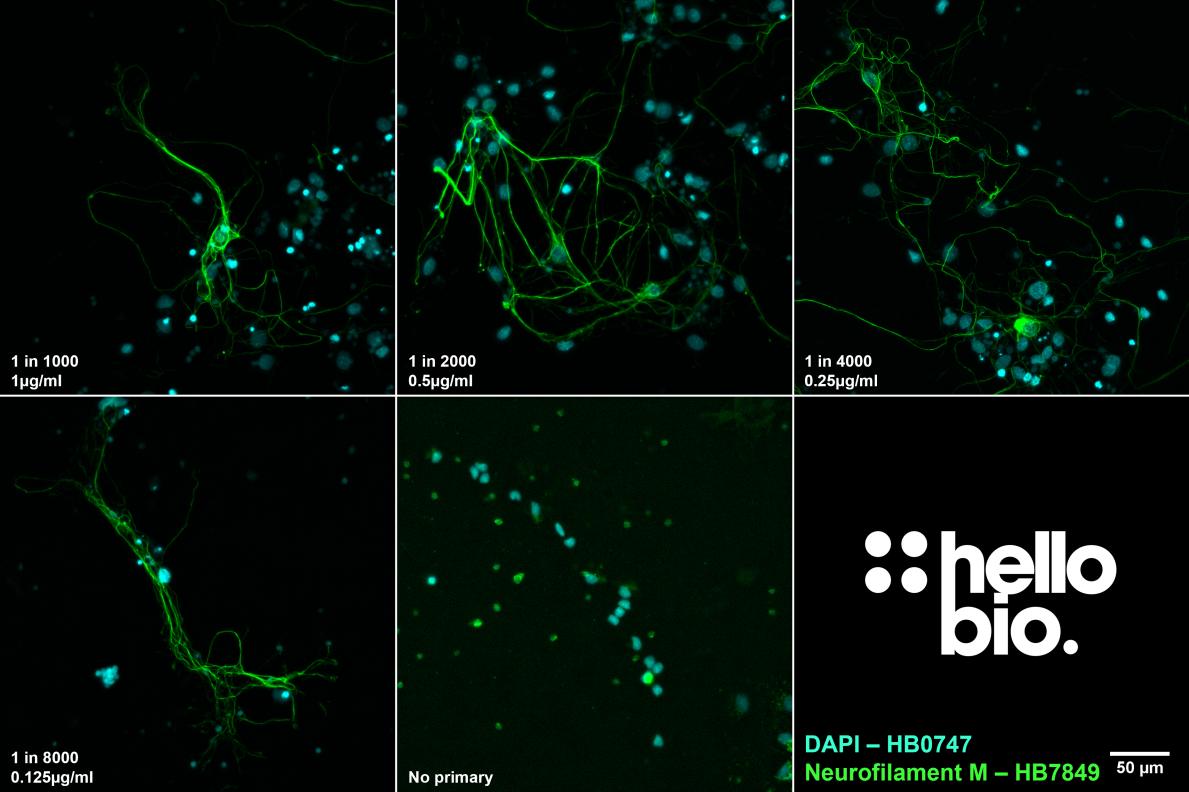
Figure 2. The effect of varying HB7849 concentration upon staining in cultured rat neurones. HB7849 produced a strong signal to noise ratio at dilutions as low as 1 in 8000 (125 ng/ml). Method: neurones were cultured from PND2 rats following established protocols (Brewer and Torricelli, 2007. Nat Protoc 2, 1490–1498) and fixed with 4% PFA on DIV21. Cells were permeabilised with 0.1% Triton X-100 followed by blocking in 1% BSA, 300mM glycine. HB7849 was incubated overnight (4°C) at dilutions ranging from 1:1000 (1µg/ml) to 1:8000 (0.125µg/ml) with a no primary step omitting the addition of HB7849 to the incubation buffer. This was followed by a one hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our ICC protocol. Images were captured using a Leica DMi8 inverted epifluorescence microscope (40x objective) in a z-stack (1µm spacing) coupled to a Leica DFC365FX monochrome digital camera with DAPI LP and FITC LP filters. Exposure times were as follows:
1:1000 – DAPI 7.9ms, FITC 50.5ms
1:2000 – DAPI 10ms, FITC 63.9ms
1:4000 – DAPI 7.5ms, FITC 50ms
1:8000 – DAPI 7.5ms, FITC 50ms
Images were processed in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682) using the subtract background (50px rolling ball radius) tool followed by Z-projection, stacking and montage creation. |
![]()
|
Figure 3. Neurofilament M expression in various tissue lysates and preparations. HB7849 revealed a single band of size 144kDa primarily present in brain cytosol fractions. Due to high levels of phosphorylation neurofilament M is well known to migrate at a significantly heavier weight than it’s predicted molecular weight in SDS-PAGE. Method: mouse brain and rat brain membrane (P2) and cytosol fractions were prepared following previous work (Molnar et al., 1993. Neuroscience 53:307-326) from freshly collected adult brains. Other tissue lysates were prepared following established protocols from freshly dissected tissue (see our guide on WB sample preparation). Samples were loaded (20µg / lane) onto a 7.5% acrylamide gel alongside a protein ladder (BioRad Precision Plus dual colour, 1610374) before being run at 60V for 30 minutes followed by 130V for 100 minutes. Wet transfer to a PVDF membrane was completed in 90 minutes using 400mA. The membrane was blocked for 2hrs in 5% non-fat dry milk before being incubated overnight at 4°C in HB7849 at a 1:5000 dilution (0.2µg/ml). Following washing the membrane was incubated in secondary antibody (1:10,000 dilution, Polyclonal goat anti-mouse HRP conjugated, Sigma Aldrich A3682) for 2hrs. For more detail please see our Western blotting protocol. Detection was accomplished using Clarity Western ECL substrate (BioRad, 1705061) and a Licor Odyssey Fc imaging system (ECL channel: 10 min exposure, 700nm channel: 30 sec exposure). |
![]()
|
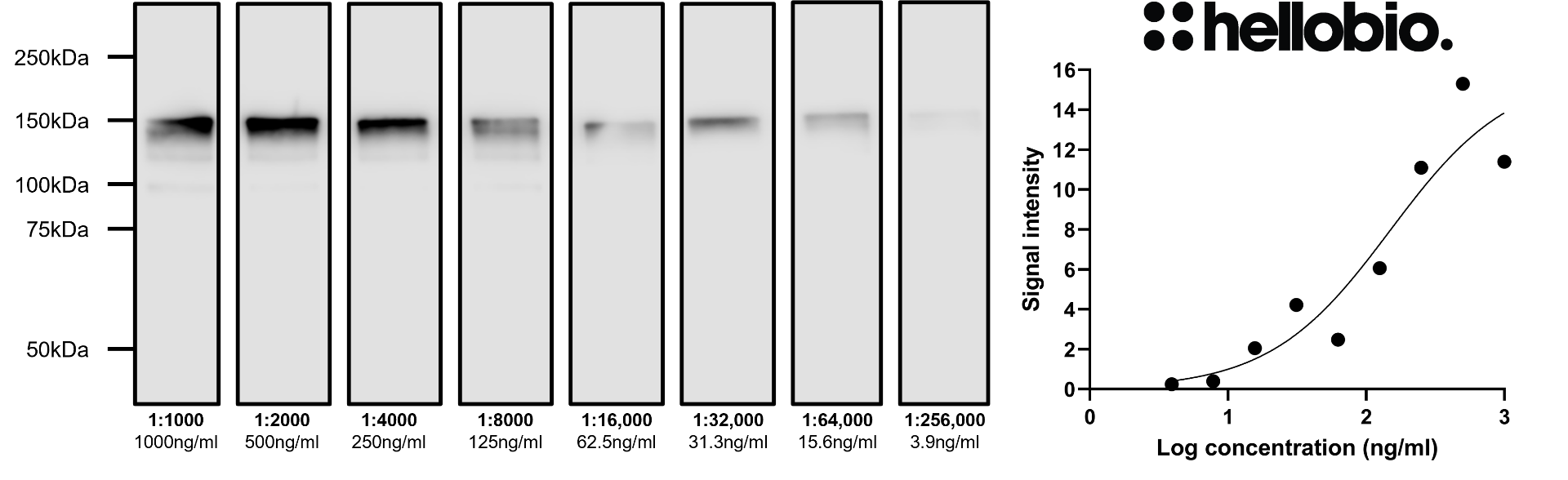
Figure 4. Concentration response of HB7849 staining in a rat brain cytosol preparation. HB7849 shows consistent results with low background at dilutions as low as 1:64,000 (6.25 ng/ml). Due to high levels of phosphorylation neurofilament M is well known to migrate at a significantly heavier weight than it’s predicted molecular weight in SDS-PAGE. Method: cytosol fractions were prepared from fresh rat brains following established protocols (Molnar et al., 1993. Neuroscience 53:307-326). Rat cytosol samples were loaded (20µg / lane) onto a 7.5% acrylamide gel alongside a protein ladder (BioRad Precision Plus dual colour, 1610374) before being run at 60V for 35 minutes followed by 130V for 90 minutes. Wet transfer to a PVDF membrane was completed in 90 minutes using 400mA. Following transfer the membrane was cut into strips using Ponceau dye to visualise and cut individual lanes. Strips were blocked for 2hrs in 5% non-fat dry milk before being incubated overnight at 4°C in HB7849. Each strip was incubated separately with a separate HB7849 concentration with this ranging from 1µg/ml (1:1000 dilution) to 3.9ng/ml (1:256,000 dilution). Following washing the membrane was incubated in secondary antibody (1:10,000 dilution, Polyclonal goat anti-mouse HRP conjugated, Sigma Aldrich A3682) for 2hrs. For more detail please see our Western blotting protocol. Detection was accomplished using Clarity Western ECL substrate (BioRad, 1705061) and a Licor Odyssey Fc imaging system (ECL channel: 10 min exposure, 700nm channel: 30 sec exposure). Band intensity was calculated using Image Studio version 5.2.5 (LiCor) and a graph was constructed in GraphPad Prism 9 using a 3-parameter Hill equation curve fit. |
![]()
|
Figure 7. Neurofilament M and GAPDH expression in various tissue lysates and preparations. HB7849 revealed a band of size 138kDa primarily present in brain cytosol fractions. Due to high levels of phosphorylation neurofilament M is well known to migrate at a significantly heavier weight than it’s predicted molecular weight in SDS-PAGE. Method: mouse brain and rat brain membrane (P2) and cytosol fractions were prepared following previous work (Molnar et al., 1993. Neuroscience 53:307-326) from freshly collected adult brains. Other tissue lysates were prepared following established protocols from freshly dissected tissue (see our guide on WB sample preparation). Samples were loaded (20µg / lane) onto a 10% acrylamide gel alongside a protein ladder (BioRad Precision Plus dual colour, 1610374) before being run at 60V for 30 minutes followed by 130V for 120 minutes. Wet transfer to a PVDF membrane was completed in 90 minutes using 400mA. The membrane was blocked for 2hrs in 5% non-fat dry milk before being incubated overnight at 4°C in HB7849 at a 1:5000 dilution (0.2µg/ml) and HB9177 at a 1:4,000 dilution (0.25 µg/ml). Following washing the membrane was incubated in secondary antibody (1:10,000 dilution, Polyclonal goat anti-mouse HRP conjugated, Sigma Aldrich A3682) for 2hrs. For more detail please see our Western blotting protocol. Detection was accomplished using Clarity Western ECL substrate (BioRad, 1705061) and a Licor Odyssey Fc imaging system (ECL channel: 10 min exposure, 700nm channel: 30 sec exposure). |
![]()
|
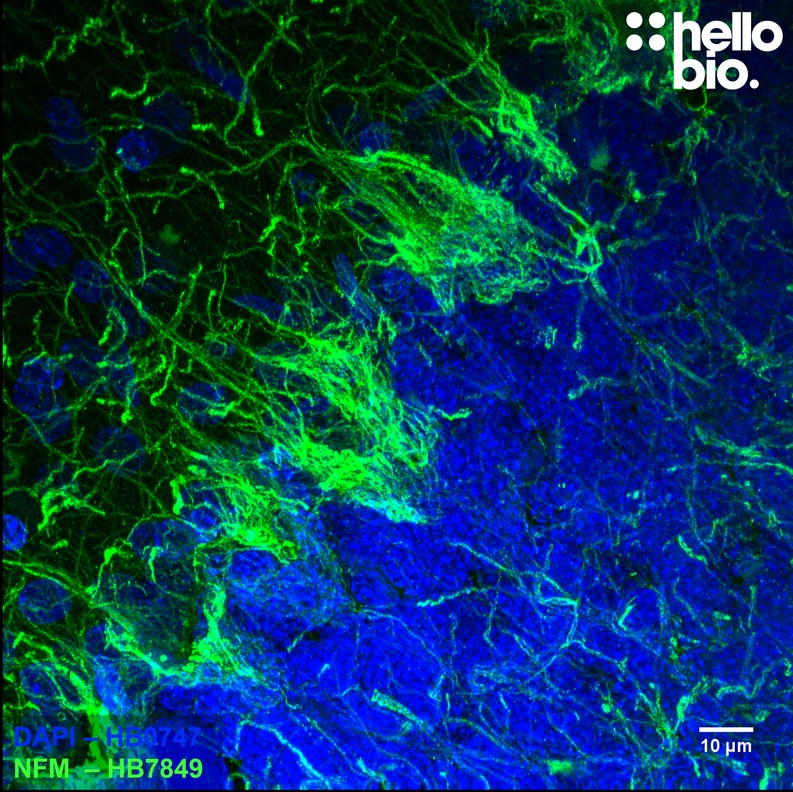
Figure 5. Neurofilament M expression in rat cerebellum. HB7849 visualised the dense network of neuronal projections present in rat cerebellum. Method: Brains were dissected from adult rats and fixed for 48hrs in 4% PFA before then incubated in 30% sucrose (in PBS) until the brains had sunk. A freezing microtome was used to cut 40µm horizontal slices before sections were incubated in 1% NaBH4 for 30 minutes then 0.05M glycine for 30 minutes. Sections were blocked in 2% BSA, 3% goat serum before incubation overnight in HB7849 (1:1000, 1µg/ml) at 4°C. This was followed by a two hour incubation with a polyclonal goat anti-mouse DyLight 488 conjugated secondary antibody (Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our IHC(IF) protocol . Images were captured using a Leica SPE confocal laser scanning microscope coupled to a Leica DMi8 inverted epifluorescence microscope. The image was captured using a 63x objective, 405nm (18.9% power, gain: 567V) and 488nm (25.6% power, gain: 779V) in a z-stack (0.59 µm spacing). Deconvolution was carried out using Huygens Essential (Scientific Volume Imagine) followed by the stack being flattened using a maximum Z projection in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682). |
![]()
|
Figure 6. Dense network of axonal projections visualised using HB7849 in rat cerebellum. HB7849 visualised the dense network of neuronal projections present in rat cerebellum. Method: Brains were dissected from adult rats and fixed for 48hrs in 4% PFA before then incubated in 30% sucrose (in PBS) until the brains had sunk. A freezing microtome was used to cut 40µm horizontal slices before sections were incubated in 1% NaBH4 for 30 minutes then 0.05M glycine for 30 minutes. Sections were blocked in 2% BSA, 3% goat serum before incubation overnight in HB7849 (1:1000, 1µg/ml) at 4°C. This was followed by a two hour incubation with a polyclonal goat anti-mouse DyLight 488 conjugated secondary antibody (Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our IHC(IF) protocol . Images were captured using a Leica SPE confocal laser scanning microscope coupled to a Leica DMi8 inverted epifluorescence microscope. The image was captured using a 40x objective, 405nm (18.9% power, gain: 530V) and 488nm (25.6% power, gain: 872V) in a z-stack (0.76 µm spacing). Deconvolution was carried out using Huygens Essential (Scientific Volume Imagine) followed by the stack being flattened using a maximum Z projection in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682). |