COVID Rapid Tests
Selection Guide & FAQ
COVID-19 Rapid Tests wanted! But which ones?! Our selection guide helps you through the jungle of different test formats, regulations, and requirements.
All our COVID-19 (SARS-CoV-2) Rapid tests are so called lateral flow immunochromatographic assays (same principle as a common pregnancy test) with an internal procedural control, and they all give you a qualitative result within 15 minutes.
They differ in application, sample type, sensitivity, and packaging, as shown below. Visit our FAQ section, if you wish more explanation on terms of concept.
COVID Antigen Tests for professional use
Frequently Asked Questions (FAQ)
Note: We don't sell any diagnostic tests or products to private individuals.
COVID Antigen Self Tests
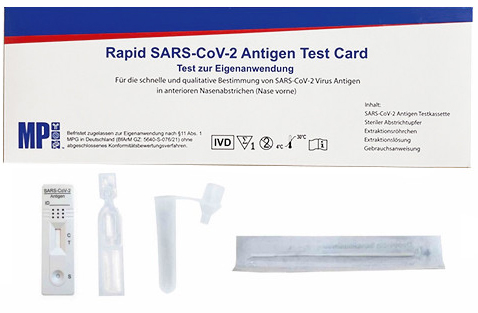 COVID Antigen Self Tests are designed to be selfapplied by the test subject. The sample is taken from the lower part of the nose with help of a (included) nasal swab. The sensitivity (reliability of the result) of correctly applied self tests corresponds to that of the professional antigen tests. We sell our self tests in packages of five or 25 single packed tests, each including all necessary components. These are convenient, if you want to supply them to your employees for home testing, f.e.
COVID Antigen Self Tests are designed to be selfapplied by the test subject. The sample is taken from the lower part of the nose with help of a (included) nasal swab. The sensitivity (reliability of the result) of correctly applied self tests corresponds to that of the professional antigen tests. We sell our self tests in packages of five or 25 single packed tests, each including all necessary components. These are convenient, if you want to supply them to your employees for home testing, f.e.
COVID Antigen Rapid Tests for professional use
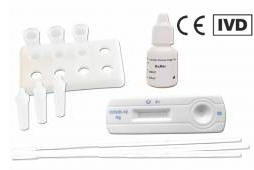 COVID Antigen Rapid Tests for professional use are diagnostic devices (CE-IVD marked). They must be applied by a trained person, which may be a doctor or pharmacist, but as well a designated staff in your company or institution, who attended a special training.
COVID Antigen Rapid Tests for professional use are diagnostic devices (CE-IVD marked). They must be applied by a trained person, which may be a doctor or pharmacist, but as well a designated staff in your company or institution, who attended a special training.
Professional Rapid Tests come as cost-efficient kits of 20 or 25 test cassettes. All needed equipment (like buffer and swabs) is included, but is not single packed. The kits are eligable, if you want to test several persons at one location (p.e. a doctor's office, pharmacy, company, or school). As sample, they require either oropharyngeal, nasopharyngeal, or nasal swab specimen, according to the product description.
Frequently Asked Questions
Do the rapid tests also detect the omicron variant of the virus?
The Paul Ehrlich Institute states:
"Die große Mehrheit der 245 Antigentests, die bis zum 14.12.2021 untersucht wurden, weisen das Nukleo-Protein (N-Protein) des Coronavirus nach. Die Mutationen der Omikron-Variante betreffen aber primär das S-Protein. Auf der Grundlage der aktuellen Datenlage geht das Paul-Ehrlich-Institut davon aus, dass die allermeisten der in Deutschland angebotenen und positiv bewerteten Antigentests eine Omikron-Infektion nachweisen können."
(The vast majority of the 245 antigen tests examined by Dec. 14, 2021, detect the nucleo-protein (N-protein) of coronavirus. However, the Omikron variant mutations primarily affect the S protein. Based on current data, the Paul Ehrlich Institute assumes that the vast majority of antigen tests offered and positively evaluated in Germany can detect an Omikron infection.)
All corona rapid tests and self-tests available from BIOZOL detect the coronavirus nucleoprotein and, based on current knowledge, should also be able to detect an omicron infection.
Are the rapid tests individually packaged? Can I give them to my employees to take home for self-testing?
Our rapid tests contain all the components required to perform the test. For the tests for professional use, the test cassettes are packaged individually, but the accessories (swabs, test tubes, buffers) are not. These tests are also not suitable for home testing because medical training (see below) is a prerequisite for use.
In the so-called self-tests, all components are packaged per individual test (test cassette with swab, test tubes and buffer). These are suitable to give along for home testing.
What does "professional use" mean, who is allowed to perform these tests?
Rapid tests for professional use are diagnostic devices (CE-IVD marked) that must be used by a trained person. This can be a physician or pharmacist, but also a designated employee of your company or institution who has attended a special training. Such medical training is offered online by the Order of St. John, for example. We are happy to act as an intermediary and will cover the costs of the training once if you purchase a professional rapid test kit from us. For more information, please contact info@biozol.de.
No special training is required for the self-tests. Anyone may use them. However, we generally sell only to companies and not to private individuals.
What kind of swab specimens do I need to take for the rapid test?
The highest viral load in infected patients is found in the throat (pharynx) and nose. Antigen tests (which directly detect components of the virus) are therefore performed using either nasal swab specimens (from the lower part of the nose) or naso- or oropharyngeal swab specimens (from the nasal and/or oral pharynx).
The swabs included in the test kit differ depending on their intended use: pharyngeal swabs cannot be used to collect samples from the nose and vice versa. Be sure to follow the product description and instructions for use of the respective test.
For our self-tests, nasal swab specimens from the lower nasal region are used. Proceed exactly according to the instructions.
Is a self-test (swab specimen from the anterior nasal region) as reliable as a professional nasopharyngeal swab test?
Self-tests have been part of national testing strategies in Germany and Austria, among others, for some time. Samples are usually taken by swabbing the anterior nasal region. Properly used, these represent a reliable alternative to professional nasopharyngeal swab tests (study by Lindner et al., 2020).
Do the cotton swabs used for swabbing contain chemicals or worms?
No! Our swabs do not contain toxic or harmful substances. The colonization of cotton swabs with worms is an unsubstantiated rumor.
What is the difference between sensitivity and specificity - and how good is good enough?
The specificity of a diagnostic test corresponds to its ability to decide between different analytes (e.g. pathogens). A test with a high specificity will reliably identify a healthy person as negative, i.e. show very few false positive results.
The sensitivity of the test corresponds to its ability to detect an infected person as such. The higher the sensitivity, the fewer false negative results the test will produce.
Both sensitivity and specificity of a rapid antigen test are validated and reported in relation to the qPCR result of the same sample. The minimum criteria for SARS-CoV-2 antigen tests, as defined by the PEI and the basis for the BfArM listing (see below), include a sensitivity >80% and a specificity >97%. All our tests are well above the required minimum values for sensitivity and specificity.
Which legal requirements must be met for the approval of Corona rapid tests in Germany?
The BfArM (German Federal Institute for Drugs and Medical Devices) provides a list of antigen tests for the direct pathogen detection of the coronavirus SARS-CoV-2, which are subject of the claim according to § 1 sentence 1 of the Coronavirus Test Regulation. It contains the corresponding tests which, according to the current knowledge of the BfArM, are in circulation in Germany and, according to the manufacturer's information, fulfill the respective current minimum criteria for antigen tests defined by the Paul Ehrlich Institute (PEI) in coordination with the Robert Koch Institute (RKI). Listing with the BfArM is a prerequisite for cost coverage by German health insurance companies.
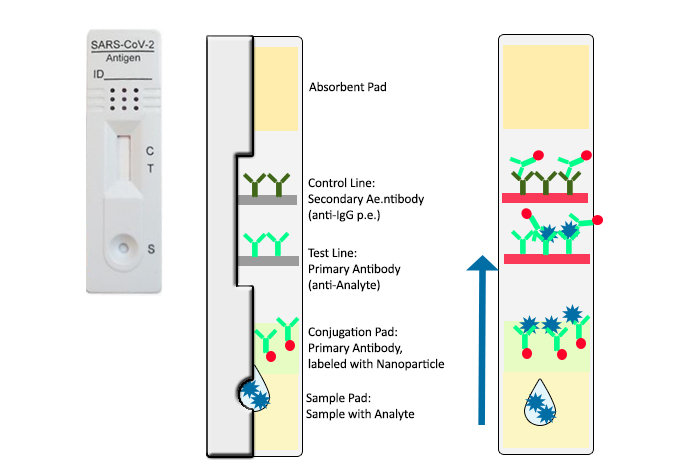
How does a lateral flow immunoassay work?
All our COVID-19 (SARS-CoV-2) Rapid tests are so called lateral flow immunochromatographic assays (same principle as a common pregnancy test) with an internal procedural control.
The analyte (SARS-CoV-2 protein) in the sample is recognized by a specific antibody. Conjugated nanoparticles make the reaction visible as a positive test line (in case of present antigen) or as control line only (in case of a sample without recognized antigen).
How does the result of a rapid test differ from that of a PCR?
Rapid antigen tests detect proteins of the virus via antibody binding. A qPCR-test detects the virus' genetic information, its RNA, which is amplificated many times during the reaction, what makes a PCR extremly sensitive. Antigen tests are cheaper, faster and easier to use, than a (lab-based) qPCR test, but also noteworthy less sensitive. A positive result of a rapid antigen test is always to be confirmed by a qPCR test. Find our qPCR tests here.
09.06.2021

New: Protein Detecti...
Your sidekick for protein staining

Cell Manipulation
MDR Approval for Medical Devices

Labortops #2
Super Sale: up to 88% off labware
Obesity and Metaboli...
Research Reagents from Rockland, GeneTex and more

Spring Promo
Up to 30 % off Antibodies and ELISA kits



