Immune Checkpoint markers
Antibodies for Immunohistochemistry
A growing number of immune checkpoints emerge as targets for anticancer therapy. So far, PD-1 and CTLA-4 emerged as the most successful immune targets for anticancer therapies. The recently discovered TIGIT pathway provides significant therapeutic promise, particularly in combination with other immune checkpoint inhibitors.
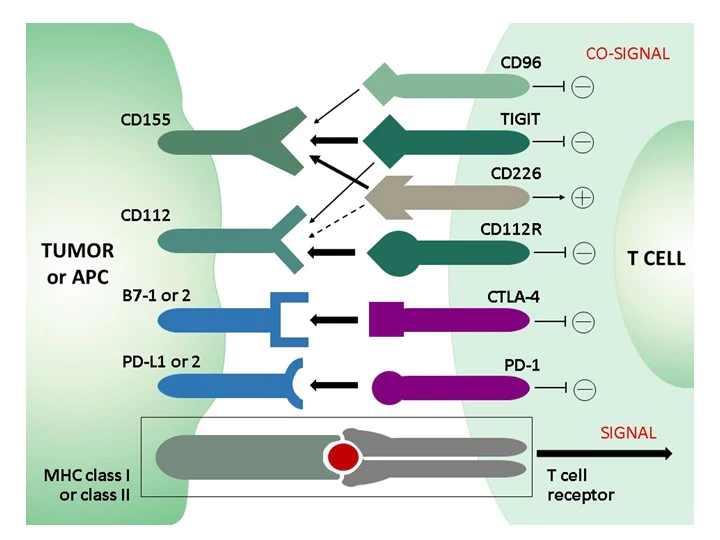 In the healthy immune system, co-inhibitory and co-stimulatory receptors on T cells regulate the stimulation of T cell receptors (TCRs) to limit unwanted autoimmune responses. Whereas in acute infection CD8 effector T cells become memory cells after clearance of the pathogen, prolonged stimulation and inflammation - either by non-self antigens in chronic infection or by self-antigens in the tumor microenvironment (TEM) in cancer - leads to a progressive loss of function of CD8 T cells, termed "T cell exhaustion." A phenotypic feature of exhausted CD8 T cells is the increased and sustained expression of different combinations of inhibitory receptors (immune checkpoints), including PD-1, STLA-4, TIM-3, LAG-3, and others, such as TIGIT or the recently discovered novel immune checkpoint CD112R. Due to the involvement of these inhibitory receptors in T-cell dysfunction, new therapeutic strategies have been developed that aim to block the inhibitory receptors with monoclonal antibodies (checkpoint blockade) and thus restore T-cell function.
In the healthy immune system, co-inhibitory and co-stimulatory receptors on T cells regulate the stimulation of T cell receptors (TCRs) to limit unwanted autoimmune responses. Whereas in acute infection CD8 effector T cells become memory cells after clearance of the pathogen, prolonged stimulation and inflammation - either by non-self antigens in chronic infection or by self-antigens in the tumor microenvironment (TEM) in cancer - leads to a progressive loss of function of CD8 T cells, termed "T cell exhaustion." A phenotypic feature of exhausted CD8 T cells is the increased and sustained expression of different combinations of inhibitory receptors (immune checkpoints), including PD-1, STLA-4, TIM-3, LAG-3, and others, such as TIGIT or the recently discovered novel immune checkpoint CD112R. Due to the involvement of these inhibitory receptors in T-cell dysfunction, new therapeutic strategies have been developed that aim to block the inhibitory receptors with monoclonal antibodies (checkpoint blockade) and thus restore T-cell function.
The continued clinical success of therapies with checkpoint inhibitors against PD-1, PD-L1, or CTLA-4 in terms of overall survival of cancer patients demonstrates the therapeutic potential of immune checkpoints and gives rise to promising new targets and combinatorial approaches for immune checkpoint blockade strategies. A recent therapeutic target is TIGIT: monoclonal antibodies against TIGIT not only blocked its inhibitory effect, but also shifted the balance in favor of the coactivating receptor CD226, which shares TIGIT ligands. TIGIT and CD226 form a signaling pathway similar to that of the co-inhibitory receptor CTLA-4 and the co-activating receptor CD28.
 For many of these immune checkpoint targets, we provide high-quality monoclonal antibodies for immunohistochemical application in standard FFPE human tumor tissues for diagnostic purposes under our brand dianova.
For many of these immune checkpoint targets, we provide high-quality monoclonal antibodies for immunohistochemical application in standard FFPE human tumor tissues for diagnostic purposes under our brand dianova.
Novel Immune Checkpoint marker TIGIT
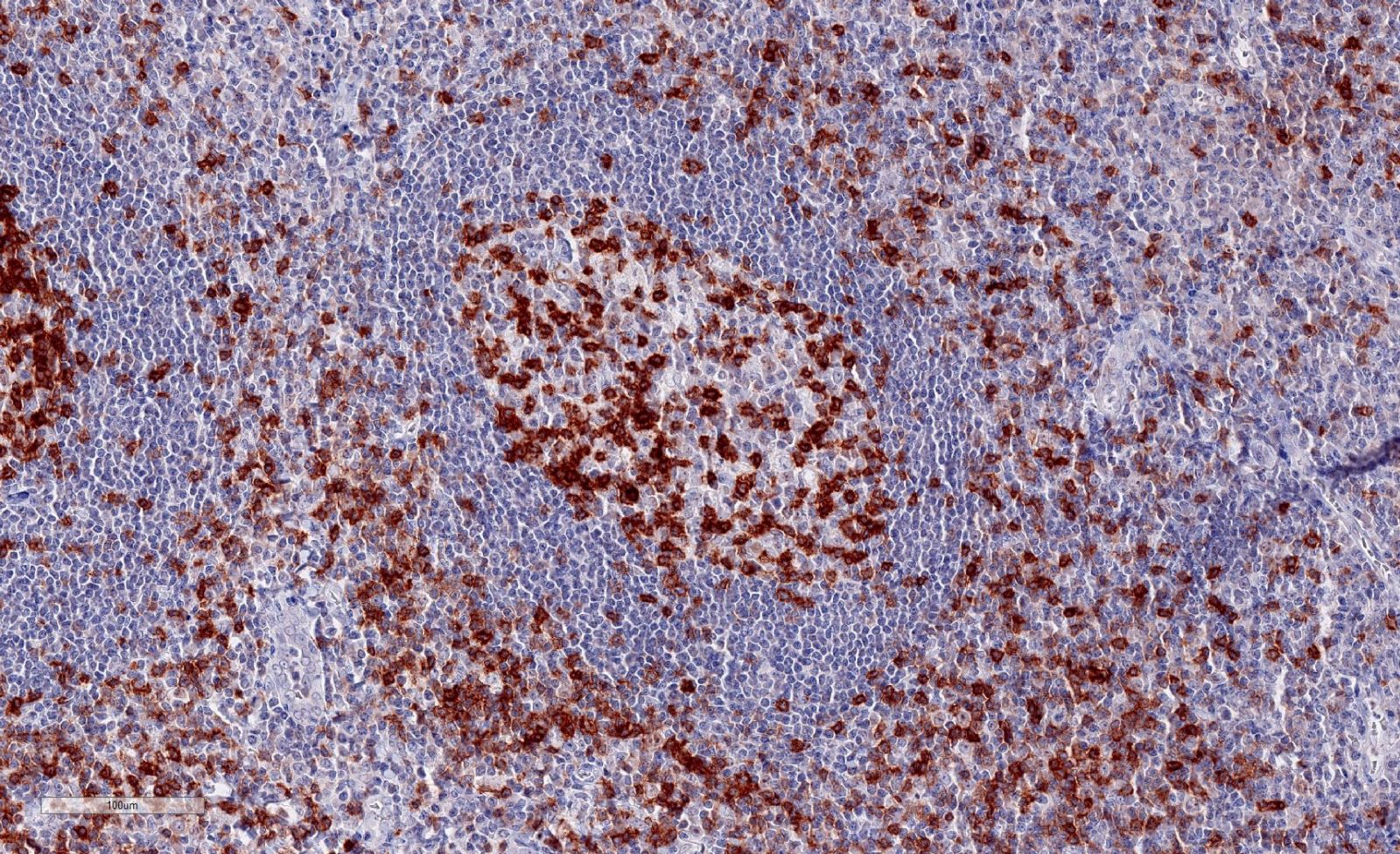 Clone TG1 is the first monoclonal antibody detecting TIGIT (T cell immunoreceptor with Ig and ITIM domains) in standard FFPE human tissue specimen. It has been validated for the identification of TIGIT positive T-cells infiltrating human tumors in order to allow the detection of TIGIT in the tumor microenvironment under pathological conditions. Immunohistochemical application of monoclonal antibody TG1 may provide valuable information for clinical research and potential therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint.
Clone TG1 is the first monoclonal antibody detecting TIGIT (T cell immunoreceptor with Ig and ITIM domains) in standard FFPE human tissue specimen. It has been validated for the identification of TIGIT positive T-cells infiltrating human tumors in order to allow the detection of TIGIT in the tumor microenvironment under pathological conditions. Immunohistochemical application of monoclonal antibody TG1 may provide valuable information for clinical research and potential therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint.
Annibali et al. published a study on the development of a new TIGIT scoring system for Hodgkin lymphoma based on the expression of TIGIT, PD-1 and PD-L1 using our dianova anti-TIGIT TG1 antibody to analyze TIGIT expression.
Niebel et al. studied the DNA methylation regulated TIGIT expression within the melanoma microenvironment as a promising marker for overall patient survival, using our dianova anti-TIGIT TG1 antibody.
Novel Immune Checkpoint marker CD112R
TIGIT and CD226 constitute a T cell co-signaling pathway in which CD226 and TIGIT, respectively, serve as costimulatory and co-inhibitory receptors for the ligands CD155 and CD112. This TIGIT signaling axis includes a complex receptor ligand system with the marker CD112R, which has become a promising target in cancer immuno-therapy. CD112R, a member of poliovirus receptor–like proteins, is preferentially expressed on T-cells and inhibits T-cell receptor mediated signals. Blockade of the CD112R-CD112 interaction enhances T cell response. CD112, the ligand for CD112R, is widely expressed on tumor cells and on antigen-presenting cells. CD112R acts as a co-inhibitory receptor for T cells by its ability to compete with CD226 to bind to CD112. Blockage of the CD112R-CD112 interaction enhances human T cell response.
Anti-PVRIG/CD112R clone R12 has been developed for detection of CD112R in routine formalin-fixed paraffin-embedded tissue specimen (IHC FFPE). Clone R12 displays no background in epithelial cells and in non lymphoid cells and has been validated for sensitivity and specificity in different normal and tumor tissues.
Selected immune checkpoint marker antibodies for immunohistochemistry:
| Art. Nr. | Product description |
|---|---|
| DNA-IHC-184010 | Monoclonal Anti-PD-L1 from Rabbit [HL1041] |
| ODN-DIA-R12 | Monoclonal Anti-CD112R/PVRIG (Hu) from Mouse [R12] |
| ODN-DIA-TG1-M | Monoclonal Anti-TIGIT (Hu) from Mouse [TG1] |
| ODN-DIA-TG2-M | Monoclonal Anti-TIGIT (Hu) from Mouse [TG2] |
This might also interest you:
>> News: Adaptive Immunity - HLA-G antibody panel for flow cytometry
>> News: Cancer Research - PathPlus™ validated antibodies from LSBio
>> Journal Club: The RANK Pathway as an Immune Modulator in Breast Cancer
26.04.2022
Complement System
WIESLAB® Functional Assays from Svar
Serum-free Organoid...
Afamin/Wnt3a Complex - now 10 % off
Uncoated ELISA Kits
The cost-saving choice - now 30% off

Microplastics
Reference Materials from LGC Standards - 20% off!
CUT&Tag-IT
Genome-wide analysis of histone marks

