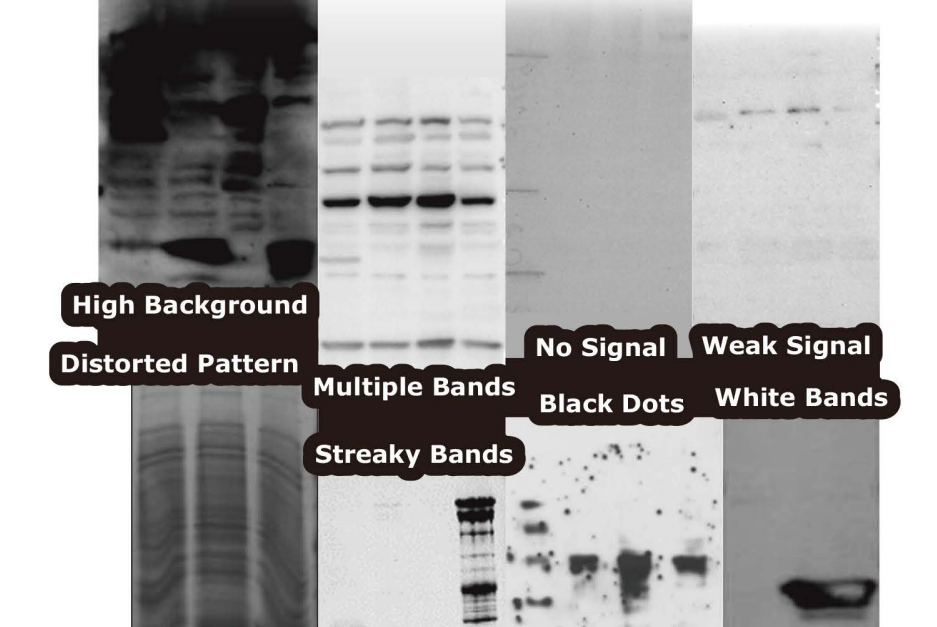
Western Blot
A comprehensive selection of troubleshooting tips

Our partner GeneTex supports researchers with a comprehensive selection of troubleshooting tips for many issues which can occur in a western blot (WB) experiment.
Download also the Western Blot Troubleshhoting Guide from GeneTex as pdf.
Be sure to load proper positive and negative controls to ensure that the WB procedure is performed correctly.
Q5: White bands on black blots
Q8: Irregular white stains on the blot
Q1: No Signal
Possible reasons:
1) Insufficient protein loading
a. Inadequate cell lysis Ensure that cell lysis and protein extraction are properly.
b. Protein degradation Always add protease inhibitors to lysis buffer prior to cell lysis and perform protein extraction on ice to avoid protein degradation.
c. Low expression of protein of interest Increasing the amount of protein extract loaded on your gel may resolve this problem. If the protein of interest is expressed in a tissue- or cell type- specific manner, be sure to choose this specific tissue or cell type for your experiments.
d. The protein of interest is enriched in a specific organelle Biochemical fractionation of subcellular compartments may be necessary to detect this type of protein.
e. Expression of the protein of interest is induced only under certain conditions Check the relevant literature to see if any treatment (e.g., starvation or chemical agents) is required to induce adequate protein expression.
2) Inadequate transfer of protein from gel to membrane
a. Incomplete transfer Make sure the PVDF membrane remains wet during the transfer. PVDF membranes must be “activated” by exposure to methanol prior to transfer. Consult the instruction manual explaining usage of PVDF membrane before the experiment. Reversible Ponceau S membrane staining is an easy step to confirm protein transfer.
b. Over-transfer Please adjust the electrical current and time frame for transfer. The conditions should be optimized according to the molecular weight of the target protein. Note that high molecular weight proteins may require a longer time to transfer.
3) Antibody hybridization and wash procedure
a. Insufficient primary or secondary antibody being used Use the recommended antibody dilutions described on the product datasheet as a starting point for your experiment. For weakly expressed proteins, it may be necessary to increase the concentration of antibody. Avoid reusing primary antibodies whenever possible.
b. Insufficient incubation time with the primary antibody A one-hour incubation at room temperature is usually sufficient for detection of most proteins. In some cases, it may be necessary to increase the incubation time (e.g., incubate overnight at 4°C).
c. Incorrect secondary antibody used Confirm that the appropriate secondary antibody is used. Select a secondary antibody directed against the specific host species and immunoglobulin type for the primary antibody (i.e., a primary antibody raised in rabbit with isotype IgG will require an anti-rabbit IgG secondary antibody). All host species and isotype information can be found on the datasheet of the primary antibody.
d. Excessive washing of the membrane Three washes of 5~10 minutes each are sufficient to wash out the non-specific binding in most cases. Avoid excessive washing of the membrane, as this may reduce the amount of primary antibody bound to the target antigen.
4) Poor activity of ECL detection reagents.
Make sure the ECL reagents have not expired. ECL reagent will lose activity over time, so always prepare the reagent immediately prior to the detection reaction.
5) Sodium azide interference.
Sodium azide (NaN3) is an inhibitor of HRP and may quench HRP activity. Ensure that there is no sodium azide in the antibody dilution buffer and thoroughly wash the membrane before the detection reaction.
Q2: Multiple or extra bands
Possible reasons:
1) Post-translational modifications to protein of interest
Post-translational modification(s) may result in multiple bands. The modified protein usually appears as a band(s) above the predicted molecular weight. Check the literature to see if there are any known modifications of the target protein.
2) Protein degradation
Protein degradation also results in multiple bands. The degraded protein is commonly seen as multiple bands below the predicted molecular weight. Ensure that protease inhibitors have been added to the protein extraction buffer. Avoid repeated freeze/thaw cycles of the cell lysate.
3) Protein multimerization
Properly boil the samples to ensure appropriate protein denaturation. Remember that freshly added dithiothreitol (DTT) or 2-mercaptoethanol (2-ME) in the sample buffer is required for the reduction of disulfide bonds.
4) Alternative splicing forms or novel proteins that share similar epitopes
Refer to literature and search on BLAST for the protein of interest. Load a recommended positive control.
5) The concentration of primary antibody is too high
Decrease the concentration of primary antibody or reduce the incubation time.
6) The concentration of secondary antibody is too high
Decrease the concentration of secondary antibody. Incubate with a secondary antibody only (without primary antibody) as a control.
7) Non-specific binding
Increase the duration of washing or increase the concentration of detergents in the wash buffer.
Q3: High background
Possible reasons:
1) The concentration of primary or secondary antibody is too high
Adjust the concentration of the primary or secondary antibody.
2) Overexposure
Decrease the time of exposure of the membrane.
3) Insufficient blocking
Increase the incubation time with blocking buffer, and ensure that an appropriate blocking buffer is being used.
4) Insufficient washing
Increase the duration of washing or increase the concentration of detergents in the wash buffer.
5) Antigens present in blocking buffer may cross-react with primary or secondary antibody
Change the blocking buffer (e.g., from non-fat milk to 3%~5% BSA or use a protein-free blocking buffer)
6) Membrane has dried out during incubation
Keep the PVDF membrane wet during incubation.
7) Improper membrane used
Select the appropriate type of membrane for your experiment (e.g., PVDF membrane is more sensitive than nitrocellulose membrane).
Q4: Smear patterns
Possible reasons:
1) Protein sample over-loading
Decrease the amount of protein loaded on the gel.
2) Poor gel preparation
Verify that the SDS-PAGE gel mix is correctly prepared and that the poured gel polymerizes completely. For gels stored at 4°C, confirm that they have not dried out.
Q5: White bands on black blots
Possible reasons:
1) The concentration of primary or second antibody is too high
Reduce the concentration of primary and/or secondary antibody.
2) The concentration of target protein is too high
Decrease the amount of purified protein or cell lysate loaded on the gel.
Q6: Black dots
Possible reasons:
1) Reagents are contaminated
Ensure that reagents are stored properly. If possible, prepare fresh reagents prior to each experiment.
2) The antibodies are binding to undissolved blocking reagent
Verify that the blocking reagent (e.g., non-fat powdered milk) is completely dissolved. If necessary, filter the blocking reagent.
Q7: Distorted bands
Possible reasons:
1) Poor gel preparation
Verify that the SDS-PAGE gel mix is correctly prepared and that the poured gel polymerizes completely. For gels stored at 4°C, confirm that they have not dried out.
2) Gel running speed is too fast
Slow the SDS-PAGE gel running speed by reducing the voltage.
3) Gel running temperature is too high
“Smiling” of migrating proteins can be caused by excessive running temperatures. To prevent this, run the gel at a lower voltage or cool the gel by running it in a cold room or on ice.
Q8: Irregular white stains on the blot
Possible reasons:
1) Air bubbles trapped in the gap between membrane and gel during transfer
Confirm that all air bubbles are removed before transfer.
2) Membrane was not completely covered by the antibody
Verify that the membrane is covered by a sufficient volume of reagents during incubation.
Q9: Distorted bands
Possible reasons:
1) Air bubbles trapped in the gap between membrane and gel during transfer
Confirm that all air bubbles are removed before transfer.
2) Membrane was not completely covered by the antibody
Verify that the membrane is covered by a sufficient volume of reagents during incubation.