AAV Reference Materials: Capsids (Vigene Bioscience)
Vigene Bioscience - Quality Control for your AAV based Gene Delivery
Full and empty capsids of different Adeno-associated Virus serotypes commonly used for gene transfer

Recombinant Adeno-associated virus (rAAV) is a widely used gene delivery tool for research and clinical applications. To be able to compare the pharmacokinetics and efficacy of rAAV, well characterized reference materials are needed for assays and calibration of internal reference materials. Reference materials (full capsids) with accurate vector genome concentration can be used in qPCR-based vector genome titrations.
AAV is also known to produce empty particles and the ratio of full to empty capsid is an important quality factor. AAV reference materials (empty capsids) are well characterized AAV empty viral particles. High quality and high purity AAV reference materials (empty capsids) can be used as controls and standard materials in assays, including HPLC, ELISA and all other relevant methods.
AAV Reference Materials (Empty Capsids)
| Order # | Description | Serotype |
|---|---|---|
| RS-AAV1-ET |
AAV1 reference materials (empty capsids), 2.26x10^12 VP/mL, 100 uL |
AAV1 |
| RS-AAV2-ET |
AAV2 reference materials (empty capsids), 1.27x10^12 VP/mL, 100 uL |
AAV2 |
| RS-AAV5-ET |
AAV5 reference materials (empty capsids), 1.46x10^12 VP/mL, 100 uL |
AAV5 |
| RS-AAV6-ET |
AAV6 reference materials (empty capsids), 1.79x10^12 VP/mL, 100 uL |
AAV6 |
| RS-AAV8-ET | AAV8 reference materials (empty capsids), 1.44x10^12 VP/mL, 100 uL | AAV8 |
| RS-AAV9-ET |
AAV9 reference materials (empty capsids), 1.76x10^12 VP/mL, 100 uL |
AAV9 |
AAV Reference Materials (Full Capsids)
| Order # | Description | Serotype |
|---|---|---|
| RS-AAV1-FL |
AAV1 reference materials (full capsids), 5.33x10^11 GC/mL, 100 uL |
AAV1 |
| RS-AAV2-FL |
AAV2 reference materials (full capsids), 1.82x10^11 GC/mL, 100 uL |
AAV2 |
| RS-AAV5-FL |
AAV5 reference materials (full capsids), 2.60x10^11 GC/mL, 100 uL |
AAV5 |
| RS-AAV6-FL |
AAV6 reference materials (full capsids), 4.10x10^11 GC/mL, 100 uL |
AAV6 |
| RS-AAV8-FL |
AAV8 reference Materials (full capsids), 7.97x10^11 GC/mL, 100 uL |
AAV8 |
| RS-AAV9-FL |
AAV9 reference materials (full capsids), 3.86x10^11 GC/mL, 100 uL |
AAV9 |
Applications for Adeno-associated Virus particles reference material
AAV - Full Capsids
- Reference materials for qPCR titer assays
- Controls/ Reference materials for full/empty characterization
- Bridging materials for in house reference materials
AAV - Empty Capsids
- Standard materials for ELISA, HPLC assays
- Reference materials for in-house standard materials
- Control viral vectors
Download additional documents on AAV gene delivery and reference material
 |
 |
Each lot of full and empty AAV capsids is extensively characterized using a wide variety of methods
Serotype specific AAV reference materials are produced using helper virus-free method, triple transient transfection. Full capsids are isolated via ultracentrifugation, followed by chromatographic purification. The viral particles are analyzed with both molecular-based assays and transmission electron microscopy (TEM) for full viral vector characterizations, including titer quantitation and full-to-empty ratio. The payload inside the AAV reference materials is CMV-GFP.
All AAV reference materials are characterized for identity and purity, viral particles per milliliter (mL), vector genomes per mL, as well as full/empty ratio. The lot sizes range from a few thousand vials to tens of thousands of vials for each serotype of AAV reference materials.
TEM Analysis of AAV Reference Materials

Fig. 1. TEM (transmission electron microscope) image of purified AAV9 reference materials. Full capsids appear as white spheres (white arrow) while empty capsids appear as spheres with black dots in the center (black arrow)
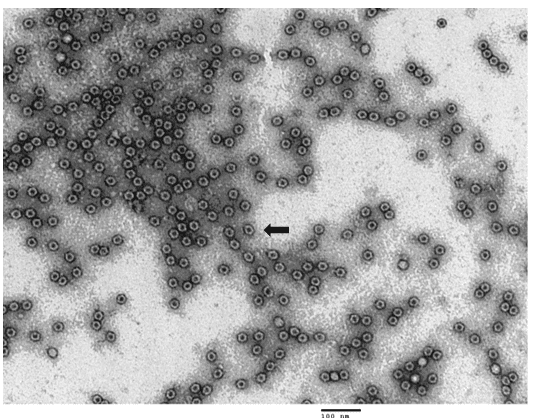
Fig.2 TEM (transmission electron microscope) image of purified AAV9 empty particles. Empty capsids appear as spheres with black dots in the center (black arrow)
Purity of AAV Reference Materials Analyzed With Silver Staining
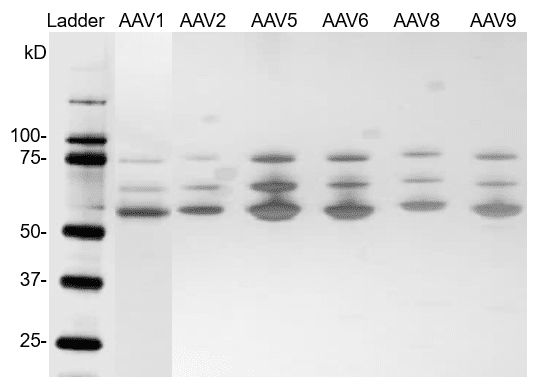
Fig. 3. Silver staining of AAV reference materials (full capsids).
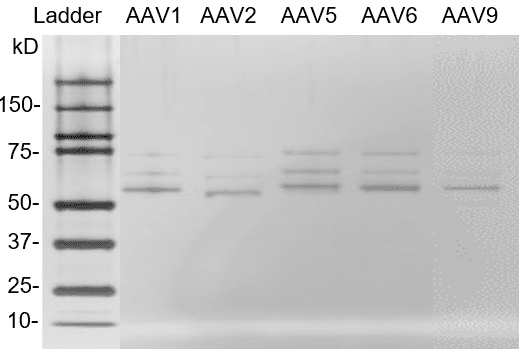
Fig. 4. Silver staining of AAV reference materials (empty capsids). AAV reference materials were loaded on a Bis-Tris SDS-PAGE gel. Proteins were detected with silver staining.
AAV reference materials were loaded on a Bis-Tris SDS-PAGE gel. Proteins were detected with silver staining.
Stability of AAV Reference Materials
The stability of AAV reference materials was demonstrated with a separate stability study of rAAV at 3E+12 GC/mL. The AAV vectors were stored at -80°C for 2 years. Viral genome titer and total particle titer did not change by qPCR and ELISA assays. The stability of the reference material (empty capsids) will be conducted in an ongoing manner. ELISA test will be performed following storage at -80°C, at 3 months, 6 months, and yearly afterwards.
15.08.2021

Neural stem cell res...
Growth factors and cytokines from Qkine

Labortops #4
Super Sale: up to 70% off labware

BIOZOL End-Of-Year P...
Up to 30 % discount on selected suppliers

iLite® Cell-Based So...
Reporter gene assays from Svar

Up to 50 % discount
Our current fall offers

